“Hardware” and “Software” of GMP – Revision for better system
1. “Hardware” and “Software” of GMP.
There are two most essential to ensure product quality, one is the equipment that can consistently be operated in a clean environment, the other is the management system by which the planned activities can be surely implemented. In short, both hardware and software should be equipped so as to comply with GMP.
Let’s look at the GMP with aspect of the Pharmaceutical Affairs Law of Japan from this viewpoint. The “Regulations for Buildings and Facilities for Pharmacies etc.”, which specified the standards for facility and equipment used in pharmaceutical manufacturing, refers to as “Hardware of GMP’. On the other hand, the “GMP Ministerial Ordinance”, which specified management system for achieving enhanced quality assurance, refers to as “Software of GMP”.
2. Revision for better system.
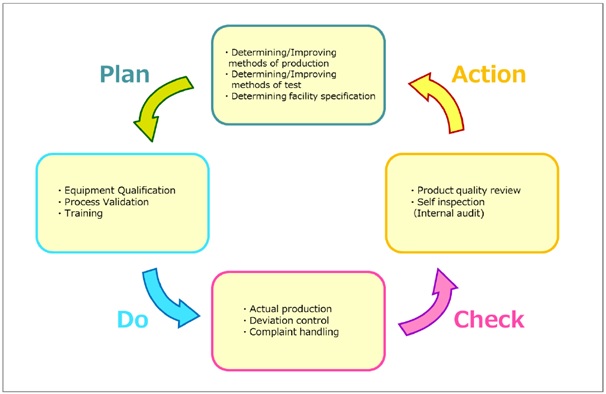
A series of processes constitute the fundamentals of pharmaceutical manufacture. These are; firstly, to document the plan pre-determined (plan); secondly, to implement it and record it accordingly (do): thirdly, to review it (check); fourthly, to improve it if it has issues (action). (See Fig.1).
The “Issues” could occur, other than such problems like non-conforming products or human errors which cause ‘deviations’ in manufacturing processes, even after marketed referring to as recalls or complaints of sold products. When those problems happen, the root causes should be investigated, and actions should be taken to prevent the recurrence. In this context, not only recurrence should be prevented by correction (corrective action), but it is also important to predict the probability of problems and prevent the occurrence (preventive action). This is called CAPA (Corrective Action and Preventive Action). In addition, self-inspection, which requires manufacturers to check the current situation according to the established procedures and record it, has been also defined in the Ministerial Ordinance on GMP. In this way, the activities for continual system reviewing should be incorporated into the established system in order to get a better quality assurance system in place.
As shown in Fig. 1 it is the cycle called a PDCA cycle, and this concept has been also incorporated into the management system established by ISO (International Organization for Standardization). ISO9001, in which essential points about management system (organization) to achieve customer satisfaction with product are summarized from a viewpoint of business management, promotes to implement PDCA cycle to achieve continual improvement. One of its features is what is also mentioned the “management responsibility”.
GMP aiming to manufacture products of high quality is a legal requirement. Though it is different from ISO, there are many common thoughts between GMP and standardized methods of quality management in ISO9000. From this background, the ICH Q10, which is a guideline to pharmaceutical quality system integrating the concepts of ISO9000 has been adopted by ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use), and notified in Japan in 2010. The ICH Q10 became something like a bridge of GMP and ICH.