FOOD BUSINESS CONDITION
Summary
I. Food business condition
To do food business must satisfy 3 conditions as below:
- There is a food business license.
- There is a certificate of food safety eligibility.
- The products must be announced on conformity with regulation on food safety.
1.1. The procedure for granting the business license with food business registration
Preparation dossier for establishment of business to be granted the business license with food business.
1.2. The procedure for granting the certificate of food safety eligibility
A dossier of application for a certificate of food safety eligibility comprises:
- An application for a certificate of food safety eligibility;
- A copy of the business license;
- Written explanations about the satisfaction of food safety and hygiene conditions of physical foundations, equipment and tools as prescribed by competent state management agencies.
- Drawing of ground design diagram of establishment and the surrounding areas;
- Diagram of food manufacturing process or process of product preservation, distribution and written explanation of physical foundations, equipment, and tools of establishment.
- Health certificates of the establishment owner and persons directly contact in food production and trading, issued by a district- or higher-level Department of Health.
- Certificates of training in knowledge about food safety and hygiene of the establishment’s owner and persons directly engaged in food production and trading.
Competence of granting the certificate:
- The Vietnam Food Administration-MOH
- Sub-department of Food Hygiene and Safety
The procedures for granting the certificates of food safety eligibility:
- Step 1: Submission dossier to The Vietnam Food Administration-MOH or the Sub-department of Food Hygiene and Safety.
- Step 2: Verification dossier: Within 05 working days as from receiving full dossiers, the agencies receiving dossiers must verify and consider the validity of dossiers and notice in writing to the establishments in case dossiers are not valid.
- Step 3: Audit at site:
After having valid result of consideration and verification of dossier, the competent agencies shall perform an audit the establishment within 10 working days. Audit content: Comparing information and verifying legality of dossier applying for grant of the certificate to original dossier archived at establishments as prescribed; verifying the condition on food safety at the establishment as prescribed and writing in the reported form.
- Step 4: In case the establishment is met on food safety as prescribed, the agency receiving dossier shall grant the certificate. In case an establishment has not yet met the requirements on food safety and must wait for completion of corrective actions, the content and time for completion must be written clearly but not exceeding 60 days.
1.3. Announcement on conformity with regulation on food safety
1.3.1 Procedures for product self-declaration:
According to item 1, article 4 Decree number 15/2018/NĐ-CP Food manufacturers and food traders shall prepare self-declaration for the products as following:
- pre-packaged processed foods
- food additives
- food processing aids
- food containers,
- Primary packages of foods
Self-declaration dossier:
1.The self-declaration form
2.Certified true copy of the food safety data sheet issued within 12 months
The self-declaration shall be posted through mass media or the producer’s website or premises; 01 copy of the self-declaration shall be submitted, directly or by post, to regulatory authority.
Right after the self-declaration is submitted, the supplier is entitled to manufacture and sell the product and assume full responsibility for the safety of such product.
1.3.2 Procedures for registration of the product declaration
According to article 6 Decree number 15/2018/NĐ-CP Food manufacturers and food traders must register the declarations of the following products:
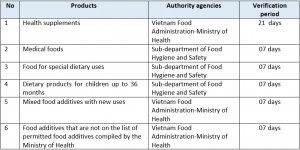
- Health supplements, medical foods, food for special dietary uses
- Dietary products for children up to 36 months
- Mixed food additives with new uses, food additives that are not on the list of permitted food additives compiled by the Ministry of Health (hereinafter referred to as “unregistered food additives”).
Application for registration of the product declaration:
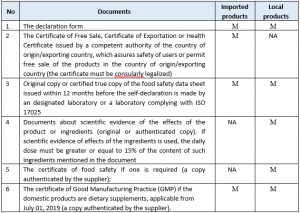
Procedures for registration of the product declaration:
II. Good manufacturing practices for health supplement
From July 01, 2019, manufacturers of dietary supplements shall satisfy GMP requirements in accordance with instructions from the Ministry of Health.
2.1. Dossier
- The application form;
- Production area and production lines layouts;
- A list of major equipment at the facility.
2.2. Procedure
- Submit dossier to Drug Administration of Vietnam;
- Within 15 working days from the receipt of the satisfactory dossier, the receiving authority shall establish an inspectorate, which will to carry out a site inspection and issue the inspection record
- If the inspection result is satisfactory, the receiving authority shall issue the certificate of GMP for dietary supplements within 30 days from the receipt of the application.
2.3. A certificate of GMP for dietary supplements is valid for 03 years from its date of issuance.